Primary Battery System Designed for Efficient Remediation of Cadmium Pollution and Power Generation Simultaneously
|
In a paper published in Fundamental Research recently, the team described how they employed zinc as the anode, graphite as the cathode, and Cd2+-contaminated media (Cd2+-contaminated water or soil) as the electrolyte to develop this system.
Cd2+, with high solubility and migration rate, is known to cause severe problems in ecology and human health. Previous technologies used to remediate Cd2+-contaminated water and soil have been constrained by high energy consumption and operation complexity, highlighting the urgent need for new remediation technologies.
The new battery system achieved efficient solidification and removal of Cd2+ in water and soil through the reduction of dissolved oxygen by internal Galvanic reactions in the battery. A large amount of OH was generated and precipitated with Cd2+ driven by an electric field.
By connecting multiple primary battery systems in series, the researchers designed an output power supply, and LEDs were continuously lit during the Cd2+ removal process.
"The idea is to kill two birds with one stone," explained CHEN Chaowen, the first author of the paper.
The researchers also checked the health of plants, zebrafish, and soil microorganisms after applying this system and found no negative effects.
The technology shows characteristics of low cost, green energy conservation, and simple operation, possessing broad application prospects.
This research was supported by the Project of the National Natural Science Foundation of China and the Plan of Anhui Major Provincial Science and Technology Project.
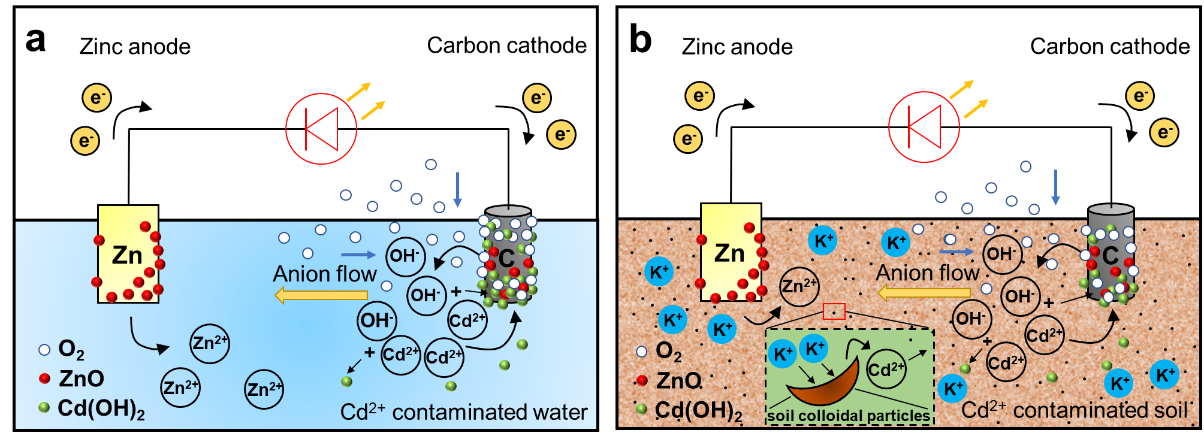
Mechanism of primary battery based on Cd2+-contaminated (a) water and (b) soil. (Image by CHEN Chaowen)
