Thanks to some possible effects, bimetallic nanoparticles can enhance electrocatalytic activity. Therefore, bimetallic nanoparticles draw considerable attention and are widely applied to electrochemical analysis and determination of heavy metal ions. However, the mechanism of electrochemical behaviors difference of bimetallic nanoparticles with different composition remains unclear.
Recently, a study team led by Prof. HUANG Xingjiu and Prof. LIU Jinhuai in Institute of Intelligent Machines (IIM), Hefei Institutes of Physical Science, preliminarily explored the electrochemical analysis mechanism toward As(III) on bimetallic nanoparticles with different composition using extended X-ray absorption fine structure (EXAFS). The research has been published in Sensors and Actuators B: Chemical. with title Electrochemical determination of arsenic(III) with ultra-highanti-interference performance using Au-Cu bimetallic nanoparticles.
Researchers synthesized Au-Cu bimetallic nanoparticles (Au93Cu7, Au89Cu11 and Au79Cu21) and then applied them as sensing materials for determination of As(III) in mild condition. The different electrochemical performance toward As(III) with different compositions of Au-Cu bimetallic nanoparticles were observed.
According to the experiment, they speculated that the Au-Au bond length (RAu-Au) might be critical to the electrochemical catalytic activity of Au-Cu bimetallic nanoparticles. The EXAFS results showed that the RAu-Au could be influenced by the amount of copper (Cu). The RAu-Au tends to become shorter with the increase of Cu concentration.
Thus, through the adjusting the stoichiometry of Au and Cu, the appropriate RAu-Au can be obtained, and the electrochemical performance in analysis of As(III) can therefore be maximized.
Based on the atomic level, the effect of mechanism of Au-Cu bimetallic nanoparticles with different composition on the electrochemical behavior was preliminarily studied. Therefore, it is anticipated that this work provides a new insight into the design of bimetallic nanostructures catalysts with high activities and further promotes their applications in toxic ions electroanalysis
This work was supported by the National Basic Research Program of China, the National High Technology Research and Development Program of China, the National Natural Science Foundation of China. The authors express thanks beamline BL14W1 (ShanghaiSynchrotron Radiation Facility) for providing the beam time.
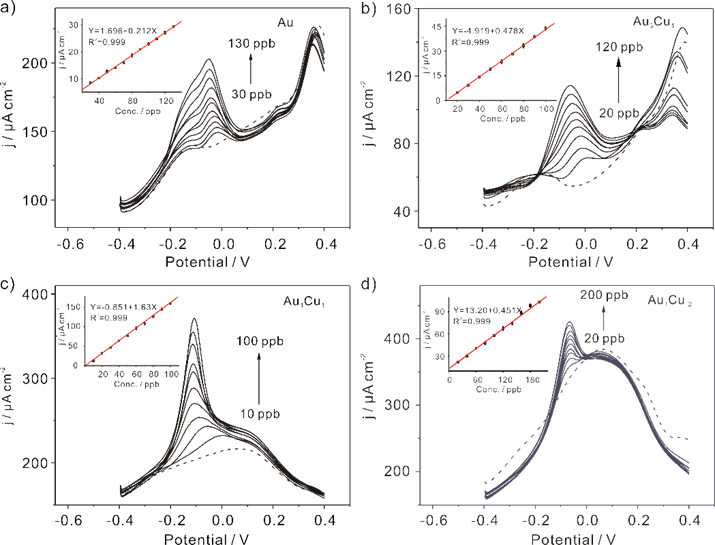
Figure 1 . Typical SWASV responses of (a) Au, (b) Au93Cu7, (c) Au89Cu11, and (d) Au79Cu21bimetallic nanoparticles modified GCE on the detection of As(III). (Imaged by YANG Meng)
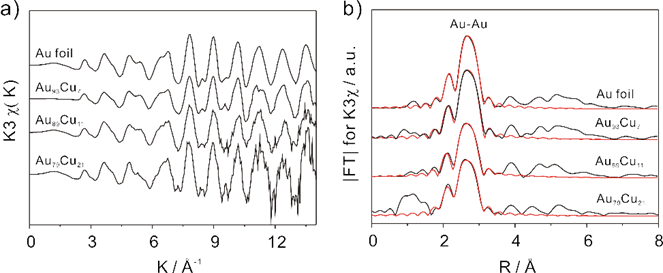
Figure 2. (a) k3-weighted Au LIII-edge experimental χ(k) data in k-space and (b) the corresponding k3-weighted Fourier transform (black line) and fitting data (red line). (Imaged by YANG Meng)
Article link:http://www.sciencedirect.com/science/article/pii/S092540051630301X
Title: Electrochemical determination of arsenic(III) with ultra-highanti-interference performance using Au-Cu bimetallic nanoparticles
Key words: Au-Cu bimetallic nanoparticles; Electrochemical determination; Arsenic(III); XAFS
Contact:
Prof. HUANG Xing-Jiu, Ph. D Principal Investigator
Institute of Intelligent Machines, Chinese Academy of Sciences, Hefei 230031, China
Tel: 86-551-6559-1167
Email: xingjiuhuang@iim.ac.cn
