Fluoride (F−) is an essential constituent for both human and animals. However, the concentration of fluoride in drinking water must be within certain limits. Excessive ingestion of fluoride will cause harmful effects, such as dental/skeletal fluorosis, fetal cerebral function and neurotransmitters. Therefore, developing efficient and cost-effective adsorbents is of great importance to the fluoride removal from contaminated water.
Recently, a research group led by Prof. LIU Jinhuai and Asso. Prof. KONG Lingtao from Institute of Intelligent Machines, Hefei Institutes of Physical Science, Chinese Academy of Sciences, has achieved important progresses in water treatment. They successfully prepared a new kind of adsorbent for fluoride removal for the first time. The novel ultralong hydroxyapatite (HAP) nanowires were demonstrated to be very effective and biocompatible adsorbents for fluoride removal from contaminated water. The study results were published in: 1. Journal of Hazardous Materials (https://www.journals.elsevier. com/journal-of-hazardous-materials/), 2. Journal of Colloid and Interface Science(https://www.journals.elsevier.com/journal-of-colloid-and-interface-science/) and 3. Chemosphere (https://www.journals.elsevier.com/chemosphere/).
In study 1, novel ultralong hydroxyapatite (HAP) nanowires were successfully prepared for fluoride removal for the first time. The maximum of adsorption capacity was 40.65 mg/gat pH 7.0 when the fluoride concentration was 200 mg/L. The thermodynamic parameters suggested that the adsorption of fluoride was a spontaneous endothermic process. Both anion exchange and electrostatic interactions were involved in the adsorption of fluoride. Furthermore, the HAP nanowires were made into HAP membrane. Membrane filtration experiments revealed that the fluoride removal capabilities depended on the membrane thickness, flow rate and initial concentration of fluoride. The filtered water amount could reach 350, 192, and 64 L/m2 when the fluoride concentrations were 4, 5 and 8 ppm, respectively, using the HAP membrane with 150 μm thickness. The effectiveness of as-synthesized ultralong HAP nanowires for fluoride removal from contaminated water was thus verified.
In study 2, Al(OH)3 nanoparticles modified hydroxyapatite (Al-HAP) nanowires were developed and made into Al-HAP membrane for fluoride removal. The maximum of adsorption capacity was 93.84 mg/g when the fluoride concentration was 200 mg/L. The adsorption mechanism was anion exchanges and electrostatic interactions. The contribution rates of HAP nanowires and Al(OH)3 nanoparticles in fluoride removal were 36.70% and 63.30%, respectively. The fixed-bed column test demonstrate that the Al-HAP was biocompatible and in a good stability during the process of water treatment. The fluoride removal abilities of Al-HAP membrane with 0.3 mm thickness could reach 1568 L/m2 when fluoride concentrations were 5 mg/L.
In study 3, a biocompatible and uniquely defined hydroxyapatite (HAP) adsorption membrane with a sandwich structure was developed for the removal of organic micropollutants for the first time. The hydrophilicity and hydrophobicity of the HAP adsorbent and membrane were tunable by controlling the surface structure of HAP. The HAP membrane could remove organic micropollutants effectively by dynamic adsorption in both aqueous and ethanol solutions. The adsorption capacities of the HAP membrane with a sandwich structure (membrane thickness was 0.3 mm) were 6700, 6510, 6310, 5960, 5490, 5230, 4980 and 4360 L/m2 for 1-naphthyl amine, 2-naphthol, bisphenol S, propranolol hydrochloride, metolachlor, ethinyl oestradiol, 2,4-dichlorophenol and bisphenol A, respectively, when the initial concentration was 3.0 mg/L. The biocompatible HAP adsorption membrane can be easily regenerated by methanol and was thus demonstrated to be a novel concept for the removal of organic micropollutants from both aqueous and organic solutions.
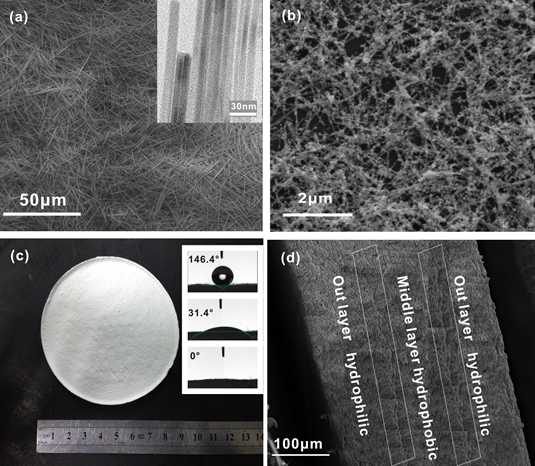
Fig. 1. (a) SEM and TEM images of the HAP nanowires; (b) SEM image of the Al-HAP nanowires; (c) Optics image and contact angles of the HAP membrane; (d) The SEM image of cross section for the HAP membrane with a sandwich structure.
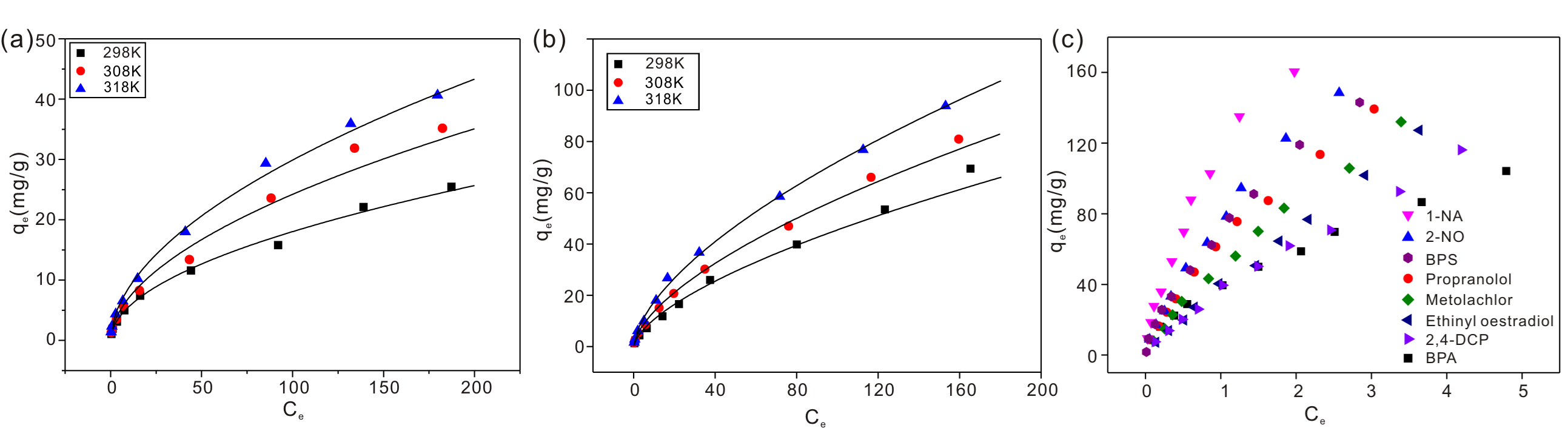
Fig. 2. (a) Fluoride adsorption isotherms on HAP nanowires at three different temperatures; (b) Fluoride adsorption isotherms on Al-HAP nanowires at three different temperatures; (c) Adsorption isotherms of the eight organic micropollutants on HAP nanowires.
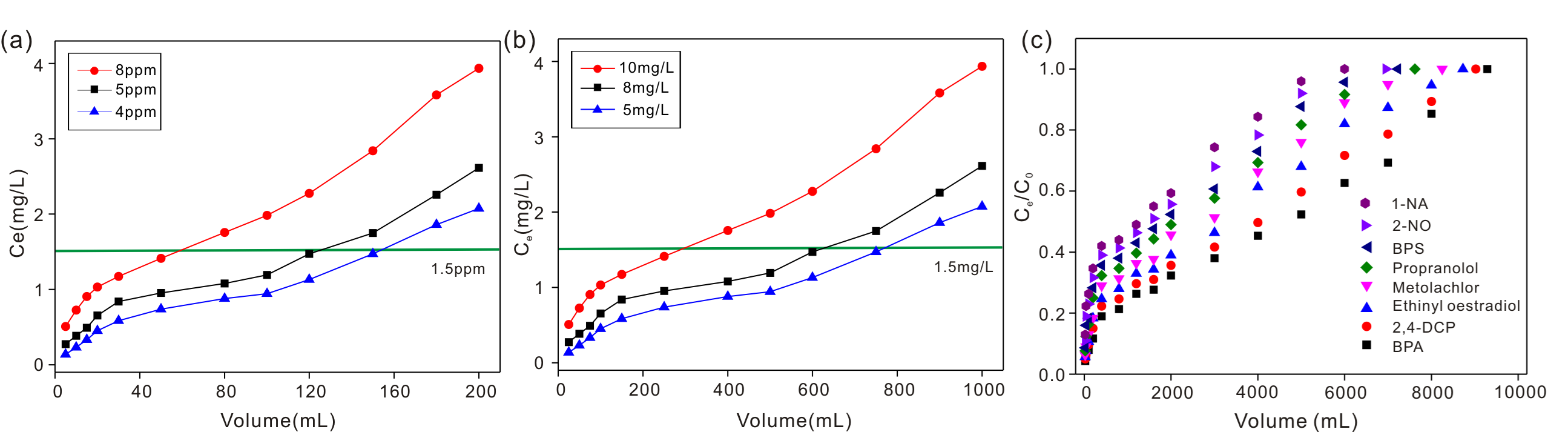
Fig. 3. (a) Adsorption behaviour of fluoride by HAP membrane; (b) Adsorption behaviour of fluoride by Al-HAP membrane; (c) Adsorption behaviour of different organic micropollutants by HAP membrane.
Article links:
http://www.sciencedirect.com/science/article/pii/S0304389415301576. (Title: Performance of novel hydroxyapatite nanowires in treatment of fluoride contaminated water)
http://www.sciencedirect.com/science/article/pii/S0021979716308712. (Title: A biocompatible and novelly-defined Al-HAP adsorption membrane for highly effective removal of fluoride from drinking water)
http://www.sciencedirect.com/science/article/pii/S0045653517301777. (Title: Study on the removal of organic micropollutants from aqueous andethanol solutions by HAP membranes with tunable hydrophilicity and hydrophobicity)
Keywords: Fluoride Removal, Hydroxyapatite (HAP) Nanowires, HAP Membranes , Al-HAP Adsorption Membrane, Organic Micropollutants
Contact:
Asso. Prof. KONG Lingtao, Institute of Intelligent Machines, Chinese Academy of Sciences (http://www.iim.cas.cn/) Hefei, Anhui 230000, China
Tel: 86-551-6559-2420
E-mail address: ltkong@iim.ac.cn
Image by HE Junyong
