Copper based nanomaterials have drawn much attention due to their own unique properties. Especially for copper selenide (Cu2Se) and copper oxide (CuO), as typical p-type semiconductors they have potential applications in the fields of thermoelectric conversion, catalysis, therapy, sensors, etc.
For porous belt-like nanomaterials, due to their higher active surface-to-volume ratios and more channels for gas diffusion in contrast to solid nanobelts. Their unique characteristics especially for gas sensing performances are further enhanced.
Accordingly, developing new strategies to prepare porous copper selenide and copper oxide nanobelts can both enrich current copper-based nanostructures and further widen their potential applications.
Recently, Prof. Guo Zheng and his co-workers in Institute of Intelligent Machines (IIM), Hefei Institutes of Physical Science, realized the synthesis of Cu2Se nanobelts and porous CuO nanobelts with highly selective sensing toward H2S. The work has been published in ACS Applied Nano Materials.( http://pubs.acs.org/journal/aanmf6)
In this work, Cu2Se nanobelts are synthesized by a facile cation-exchange method at room temperature, and then a thermal-oxidization approach realize the synthesis of porous CuO. As expected, the sensor based on porous CuO nanobelts film achieves high selective sensing toward H2S.
Obviously, porous CuO nanobelts exhibit highly selective sensing toward H2S with a low detection limit less than 10 ppb. Moreover, the researchers also present a good sensing repeatability, short response and recovery time.
Expectedly, the developed two-step approach can be extended to prepare other porous semiconductor metal oxide nanobelts for the fabrication of high-performance gas nanosensors.
This work was supported by the National Natural Science Foundation of China (Grant No. 61474122, 61774159, and 61573334), the CASHIPS Director’s Fund (Grant No. YZJJ201701) and the CAS Interdisciplinary Innovation Team of the Chinese Academy of Sciences.
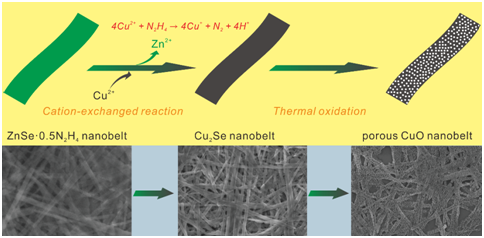
Figure 1.Schematic illumination of cation-exchange synthesis of Cu2Se nanobelts from ZnSe·0.5N2H4 precursor nanobelts and their thermal conversion to porous CuO nanobelts.(Image provided by SU Yao)
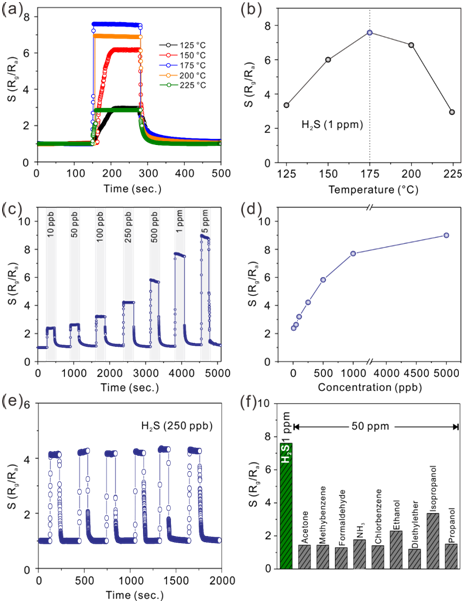
Figure 2.Gas-sensing performance studies.(a) Response curves of as-prepared porous CuO nanobelts toward 1 ppm of H2S at different working temperatures, (b) The relationship between the relative response and working temperature, (c) Real-time responses curves toward different concentrations of H2S at the optimal working temperature of 175 ˚C, (d) The relationship between the relative response and different concentrations of H2S at the working temperature of 175 ˚C,(e) Six-cycled response curves of porous CuO nanobelts towards 250 ppb of H2S at 175 ˚C, and (f) the relative responses of porous CuO nanobelts toward 1 ppm of H2S and 50 ppm of other gases (NH3, acetone, methylbenzene, formaldehyde, chlorobenzene, ethanol, diethylether, isopropanol, and propanol) at 175 ˚C.(Image provided by SU Yao)
Article link: http://pubs.acs.org/doi/10.1021/acsanm.7b00106
Title: Cation-exchange synthesis of Cu2Se nanobelts and thermal conversion to porous CuO nanobelts with highly selective sensing toward H2S
Key words: cation-exchange; copper selenide; porous; nanobelt; copper oxide
Prof. GUO Zheng, Ph. D Principal Investigator
Key Laboratory of Environmental Optics and Technology, Institute of Intelligent Machines, Chinese Academy of Sciences
Hefei 230031, China
Tel: 86-551-6559-1167
Email: zhguo@iim.ac.cn
