High electrochemical sensitivity and mutual interference behaviors have attracted broad attention in the field of electrochemical detection of heavy metal ions. Mutual interference is a severe issue that occurs during the electrochemical detection of heavy metal ions. This limitation presents a notable drawback for its high sensitivity to specific targets.
In the paper published in Analytical Chemistry (http://pubs.acs.org/journal/ancham), the search group jointly led by Prof. HUANG Xingjiu from Institute of Intelligent Machines, prepared TiO2−x Nanosheets for high electrochemical sensitivity and demonstrated an electron-induced mutual interference effect toward heavy metal ions using X-ray absorption fine structure spectra.
The researchers described the high electrochemical sensitivity and the electron-induced mutual interference effect of TiO2−x nanosheets toward heavy metal ions including Cu(II) and Cd(II)). They used Raman, ESR, XPS, Mott−Schottky, and SWASV results to determine the high-energy (001) facet percentage, oxygen vacancy concentration, surface –OH content, and charge carrier density of the TiO2−x nanosheets. These results show a synergistic effect on electrochemical sensitivity. The reduced (001) facet percentage and the low-crystallinity caused by the oxygen vacancy, as well as the high electron exchange resistance, resulted in a low catalysis activity as confirmed by surface –OH content.
They investigated the electron-induced interference effect of Cd(II) toward Cu(II) investigated by ESR, XPS, and XAFS and found that the Cd(II) promoted the free electron transfer from the defective surface of TiO2−x nanosheets to Cu(II). In addition, the Cd(II) elongated the interatomic distance between Cu and O of TiO2−x, leading to a reduced desorption energy of Cu(II) on the electrode surface.
These findings support the promising opportunity for developing highly sensitive materials and for demonstrating the mutual interference mechanism among ions during the electrochemical analysis.
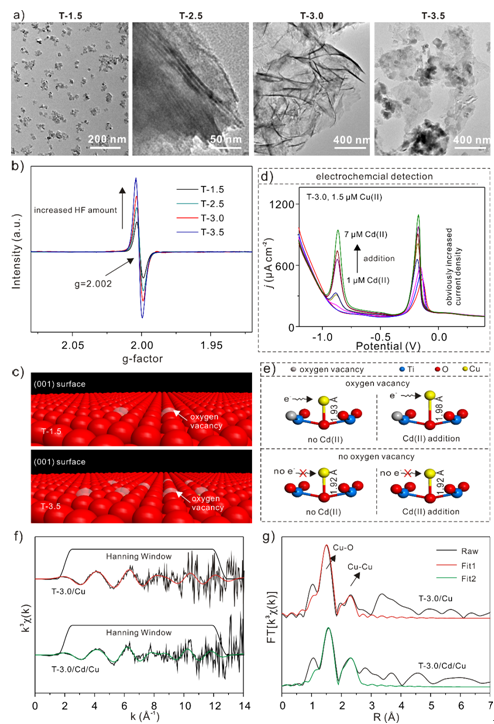
Figure.(a) TEM images of TiO2−x nanosheets synthesized with various amounts of HF amount (1.5 to 3.5 mL).(b) ESR spectra of T-1.5, T-2.5, T-3.0, and T-3.5, demonstrating the change in oxygen vacancy concentration. (c) Illustration of oxygen vacancy in two limiting cases (T-1.5 and T-3.5).(d) Stripping current density of T-3.0 toward Cu(II) with increasing Cd(II) concentration. (e) Illustration of Cd(II) electron-induced interference effect toward Cu(II).(f) Normalized, k3-weighted Cu K-XAFS spectra of the T-3.0/Cu and T-3.0/Cd/Cu. (g) Corresponding FTed k3-weighted χ(k).
Article link:https://pubs.acs.org/doi/10.1021/acs.analchem.7b02315
Title:High Electrochemical Sensitivity of TiO2−x Nanosheets and an Electron-Induced Mutual Interference Effect toward Heavy Metal Ions Demonstrated Using X-Ray Absorption Fine Structure Spectra
Keywords: TiO2−x nanosheets; electrochemical sensitivity;mutual interference; Electrochemistry
Contact:
Prof. HUANG Xingjiu, Ph. D
Institute of Intelligent Machines, Chinese Academy of Sciences, Hefei 230031, China
Tel: 86-551-6559-1167
Email: xingjiuhuang@iim.ac.cn
