After participating in the metabolic process, most of the antibiotics are discharged into the environment in the form of secondary metabolites or their original states, which  poses a
poses a  potential
potential  risk to the aquatic life and environment.
risk to the aquatic life and environment.
Fenton system is one of the most popular methods to eliminate the antibiotics in aquatic environment. However, the existing Fenton system is limited to the narrow pH range (~3-5).
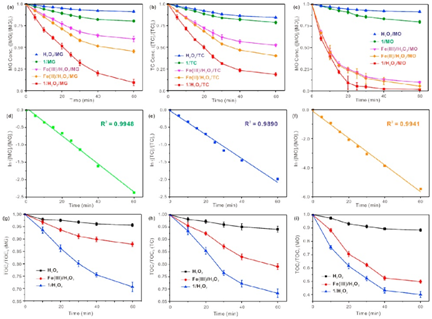
Recently, the scientific team led by Prof. KONG Lingtao from Institute of Intelligent Machines (IIM), Hefei Institutes of Physical Science, tackled the problems for high strength tetracycline, malachite green and methyl orange by a nanoscale “yarn ball”- like heteropoly blue catalyst Mg2Ti6Mo23O119SiW12 (1). The findings are published in Journal of Environmental Management(https://www.journals.elsevier.com/journal-of-environmental-management/). ( A nanoscale “yarn ball”-like heteropoly blue catalyst for extremely efficient elimination of antibiotics and dyes).
More than 90% of antibiotics and dyes are degraded within 60 min. Electron spin resonance (ESR) experiments and UV-vis spectra confirmed that the catalytic mechanisms of (1) could mainly ascribe to the (1)/H2O2 process and the possible photocatalytic oxidation of adsorbed H2O by holes (h+) in the valence band(VB) of (1)surfacegenerated·OH for extremely efficient degradation of organic contaminants.
The research widens the optimal pH values up to neutral condition and it’s significant for the expansion of the heterogeneous Fenton-like catalyst family and its application in the field of water treatment.